A Study of Blood Pressure in Relation to the Light/Dark Variations of the Cardiac Hormone Atrial Natriuretic Peptide and the Pineal Hormone Melatonin in Adult and Old Population
Paolo Lissoni1*, Antonio Bastone1, Arianna Lissoni1, Franco Rovelli1, Giuseppe Di Fede1
1Institute of Biological Medicine, Milan, Italy
Abstract
Several clinical studies have shown that blood pressure (BP) declines during the night in the healthy subjects, and that BP circadian rhythm tends to disappear with age. The mechanisms responsible for BP circadian rhythm and its aging-dependent loss need to be further understood. At present, it is already known that the two main hormones provided by hypotensive activity, consisting of the cardiac hormone atrial natriuretic peptide (ANP) and the pineal indole hormone melatonin (MLT), are mainly produced during the night, whereas hypertensive hormones, such as cortisol, are mainly produced during the early period of light phase. Then, the circadian variations of BP would be the consequence of changes in the neuroendocrine system. On this basis, a preliminary study was performed to establish which relation may exist among BP, ANP and MLT rhythms in the healthy subjects. The study included 20 65-year younger, and 20 65-year older healthy subjects. In 65-year younger subjects, both systolic and diastolic BP mean values significantly decreased during the night, whereas no significant difference occurred in the 65-older ones, because of BP values decreased in the night only in 13/20 (65) subjects. In addition, within the 65-year older group, both ANP and MLT night mean values were significantly higher in subjects with BP rhythm than in those, who had no BP daily variations. These preliminary results would suggest that age-dependent loss in the circadian rhythm of BP may be caused by the concomitant loss in the circadian secretion of at least two major hypotensive hormones, such as ANP and MLT.
Introduction
The recent advances in the knowledgement of the Chronobiology have demonstrated that most biological functions, including metabolic, endocrine, immune and vascular functions, are characterized by circadian variations during the 24-hour photoperiod, with daily rhythms related to sleep/awake or to light/dark periods1,2. The regulation of the biological rhythms would depend on several psychoneuroendocrine interactions, within them the pineal gland plays an essential role in the Chronobiology of living organisms, namely through the circadian release of its most investigated hormone, the indole melatonin (MLT), which is namely secreted during the night, with the consequent existence of a well-defined light/dark circadian rhythm in MLT production3. The cardiovascular system, which was considered as regulated by only hemodynamic mechanisms until few years ago, has also appeared to be under a physiological regulation, exerted by both psychoneuroendocrine4 and immune systems5. One of the main cardiac hormones involved in the endocrine control of heart function is the atrial natriuretic peptide (ANP), has also been proven, as well as MLT3,6, to present a circadian rhythm of its secretion, with higher values during the night and lower concentrations during the day7. Finally, it has been demonstrated the existence of a positive feed-back mechanism between ANP and MLT, with reciprocal stimulation of MLT on ANP release, and of ANP on MLT secretion8. Blood pressure (BP) itself has appeared to show a circadian rhythm, with a physiological decline in both systolic and diastolic values9. The circadian variations of BP tends to disappear with age10, as well as MLT rhythm3, and the human age would substantially consists of a progressive lack of the overall biological rhythms. The mechanisms responsible for age-related loss of nocturnal decline in BP values are complex, and they would involve several neuroendocrine interactions, since some hormones, including catecholamines11 and cortisol12, may enhance BP values, whereas other hormones, such as MLT3 and ANP7, may exert a hypotensive activity. Then, alterations in MLT and ANP secretions during the night could be involved in part in determining the progressive loss of the physiological nocturnal decline in BP values. In particular, the progressive decline with age in MLT night concentrations3 could be responsible at least in part for the aging-dependent disappearance of daily rhythm in BP values. On these bases, the present study was performed in an attempt to investigate age related changes in BP rhythm, and to analyze which relation may exist between BP rhythm and circadian secretion of the pineal hormone MLT and the cardiac hormone ANP.
Patients and Methods
The study included 40 consecutive healthy volunteers, 20 of whom were 65-year younger (M/F: 11/9; median age: 52 years, range 47-59), while the other 20 were 65-year older subjects (M/F: 8/12; median age 78 years, range 68-81). The BP values considered far each single subject was the mean of the values obtained at 15-minute intervals during the daily (from 6.00 A.M. to 10.00 P.M.) and the nightly phase (from 10.00 P.M. to 6.00 A.M.) of the photoperiod. BP values were measured in a hospital setting by a non-invasive ambulatory monitor (TM 2420, Takeda, Japan). Moreover, to evaluate MLT and ANP secretions, various blood samples were collected at 8.00 A.M., 12.00 A.M., 8.00 P.M., and at 1.00 A.M. The study was performed in summer. Serum levels of ANP and serum levels of MLT were measured in duplicate by commercial kits (ANP Ria method, Immunotechnology Service, Production BV, Wijchen, Holland; MLT, double Ab RIA method, after diethylether extraction, EuroDiagnostic- Apeldoorn, Holland). Data were reported as mean +/- SE, and statistically analyzed by the Student’s t test, the chi-square test, and the analysis of variance, as appropriate.
Results
Day and night mean values of BP observed in healthy volunteers younger and older than 65 years are illustrated in Figures 1. Both systolic and diastolic BP mean values observed during the day in 65-year younger healthy subjects were significantly higher with respect to those found during the night ( systolic BP: p< 0.005; diastolic BP: P< 0.01), whereas no significant difference was seen in the 65-year older subjects between night and day values of BP. In more detail, both systolic and diastolic BP decreased during the night in all 65-year younger subjects, and in only 13/20 (65%) 65-year older subjects. A nocturnal increase in both MLT and ANP levels occurred in all 65-year younger subjects. On the contrary, a night increase in MLT and ANP blood concentrations was seen in only 8/20 (40%) and 12/20 (60%) subjects, respectively. Figure 2 illustrates the circadian rhythm of MLT and ANP observed in 65-year older patients with or without a physiological nocturnal decline in BP values. ANP mean blood concentrations observed during the night were significantly higher in subjects with BP nocturnal decrease than in those, who had no BP circadian variation (p< 0.05). Nocturnal mean levels of MLT were also higher in subjects with than in those without BP decline during the night, even though the difference was not statistically significant.
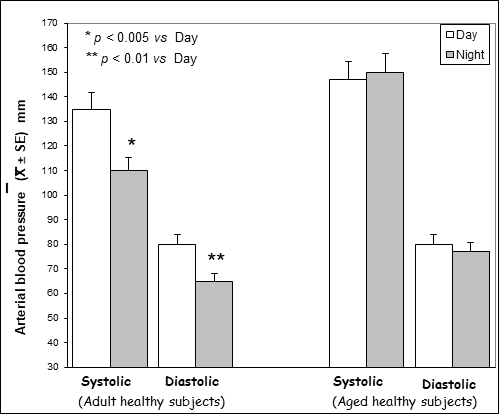
Figure 1: Day and night mean values of blood pressure in adult / aged healthy subjects.
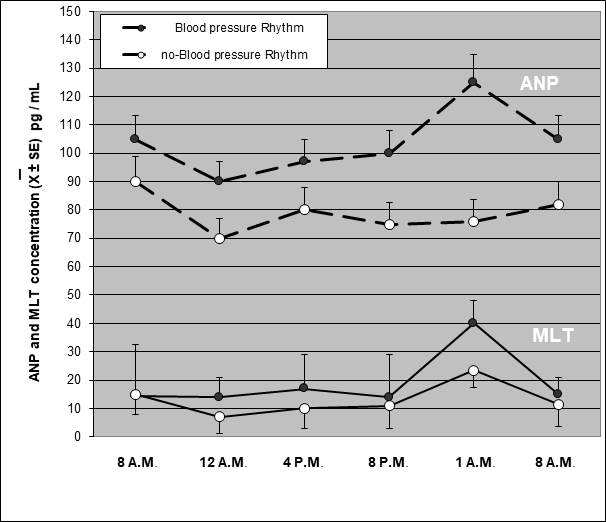
Figure 2: Circadian rhythm of melatonin (MLT) and atrial natriuretic peptide (ANP) in relation to blood pressure rhythm in a group of aged healthy subjects.
According to other authors9-12, this study confirms that BP values are characterized by circadian variations, with lower values during the night, in the healthy subjects, and that BP daily rhythm tends to disappear with age. Moreover, this study shows that age-related loss of BP circadian rhythm tends to be associated with a concomitant loss in the physiological nocturnal increase in MLT and ANP blood levels. Then, because of the hypotensive properties of both ANP and MLT3,7, the loss of the physiological nocturnal decline in BP values might depend at least in part and the concomitant failure in ANR and MLT night increase. This statement is also justified by the evidence that MLT and ANP secretions are connected by a reciprocal stimulatory action8. In particular, it has been suggested that age-related decline in the pineal endocrine activity would play a role in the enhanced frequency of cardiovascular and neoplastic diseases with age13,14. This statement is also justified by the fact that the immune secretions of cytokines, which have been proven to influence not only the immune responses, but also BP and cardiac functions, have appeared to be under a physiological central regulation played by the pineal gland15. If further studies will demonstrate that the alterations of MLT and ANP circadian rhythms may play a role in promoting the onset of a hypertensive status, the reinduction of ANP and MLT rhythms with their night increase through a nocturnal exogenous administration could counteract the onset of the essential hypertension. The present study would simply represent a preliminary clinical investigation in an attempt to better understand the mechanisms involved in the neuroendocrine control of blood pressure, and to identify new possible endocrine alterations responsible for the pathogenesis of the idiopathic hypertension. Further studies in a greater number of subjects, including the young population, and a more adequate statistical analysis, such as ANOVA test, will be necessary to better establish which relation may exist between blood pressure values and the endocrine activity of the heart and pineal gland during the light/dark photoperiod.
References
- Halberg E. Chronobiology. Ann rev Physiol. 1969; 31: 675-681.
- Moore-Ede MC, Czeisler CA, Richardson GS. Circadian timekeeping in health and disease. N EngI i Med. 1983; 309: 469-472.
- Axelrod J. The pineal gland: a neurochemical transducer. Science. 1974; 184: 1341-1348.
- Pickering TG. Sleep, circadian rhythms, and cardiovascular disease. Cardiovasc Rev Rep. 1980; 1: 37-47.
- Haus E, Lakatua Di, Swoyer i, et al. Chronobiology in hematology and immunology. Ami Anat. 1983; 168: 467-472.
- Arendt J. Melatonin. Clin Endocrinoi. 1988; 29: 205-209.
- Kenneth G. Physiology and pathophysiology of atrial peptides. Ami Physiol. 1988; 254: E1-E5.
- Lisson P, Pelizzoni F, Grugni G, et al. Melatonin response to atrial natriuretic peptide administration in healthy volunteers. J Cardiol Pharmacol. 1990; 16: 850-852.
- Pichering TG. The clinical significance of diurnal blood pressure variations. Dippers and non dippers. Circulation. 1990; 81: 700-702.
- Shimada K, Kawamoto A, Matsubayashi K, et al. Silent cereBPovascular disease in the elderly. Correlation with ambulatory pressure. Hypertension. 1990; 167: 692-699.
- Messer EH, Glase LB, Ventura HO. Diurnal variations of circadian rhythm, arterial pressure, and urinary catecholamines in borderline and established essential hypertension. Am Hearti. 1982; 104: 109-114.
- Axelrod L. Glucocorticoid therapy. Medicine. 1976; 55: 39-46.
- BPzezinski A. Melatonin in humans. N Engi J Med. 1997; 336: 186-195.
- Lissoni P, Bastone A, Pensato S, et al. A study of individual behaviour in age-related decline in the pineal secretion of melatonin: possible implications in the prevention of age-related human diseases. MOJ Lymphol Phlebol. 2018; 2: 41-43.
- Lissoni P. The pineal gland as a central regulator of cytokine network. Neurondocrinol Lett. 1999; 20: 343-349.
