Hypothalamic Inflammation and Hypothalamic Obesity: Case Report and Mini-Review
Sigridur Fjalldal1, Lars Stenberg2, Sanaz Gabery3, Sven Karlsson4, Eva Marie Erfurth4*
1Department of Internal Medicine, Helsingborg Hospital, Sweden
2Department of Diagnostic Radiology, Skåne University Hospital, Lund, Sweden
3Translational Neuroendocrine Research Unit, Department of Experimental Medical Science, Lund University, Lund, Sweden
4Department of Endocrinology, Skåne University Hospital, Lund, Sweden
Abstract
Context: Autoimmune hypothalamitis (AHT) is extremely rare and appears on the Magnetic resonance Imaging (MRI) as a predominant suprasellar mass. There are only a few case reports of AHT in the literature and none was presented together with hypothalamic obesity (HO). Methods of prediction of hypothalamus (HT) damage are included as the pathogenesis of HO.
Case description: A 19-year-old female was operated with a transcranial operation due to a large suprasellar mass with symptoms of visual deficiencies and headache. The diagnosis of AHT was confirmed in specimen of lymphocyte-dominated infiltration with plasma cells, neutrophils and fibrocytes. The operation reduced the AHT mass and a further reduction was accomplished by long-term oral Prednisone. Directly after operation she suffered from hypothalamic obesity which worsened after the introduction of Prednisone treatment. During Prednisone treatment a reduction in the inflammation mass and improvement of vision was recorded. She was treated with glucagon-like peptide 1 receptor agonist and Metformin as treatment for her diabetes mellitus type II and with supplementations of her pituitary insufficiencies. The patient exhibits dramatic weight reduction while on a strict caloric diet at two follow-up occasions. However, this effect was reversed once the patient had stopped the calorie restricted diet.
Conclusion: The coexistence of AHT and HO is extremely difficult to treat as the former involves Prednisone treatment. Pharmacological intervention of HO has had limited effect on weight, but extreme diet was successful, however with short endurance.
Introduction
Autoimmune hypophysitis (AH) is a chronic inflammation that involves the pituitary gland with a prevalence of 0.3-0.8 %1. Autoimmune hypothalamitis (AHT) is extremely rare2 and typically appears as a predominant suprasellar mass. Whether AH and AHT represent different diseases or different aspects of the same disease still remains unclear2. Patients with AHT may experience symptoms such as headache, visual disturbance, diabetes insipidus and other hypopituitary symptoms. The symptoms can be relieved with either single treatment of high dose Prednisone or in combination with the immune-suppressor azathioprine2. A possible mechanism is the presentation of autoimmune lymphocytic hypofysitis, in which inflammation of the pituitary gland takes place and spreads to the hypothalamic area5. Thus, hypothalamitis may be considered as a subtype of hypophysitis, with the same therapeutic scheme and prognosis2. Although antibodies (Ab) have been found in hypophysitis, many tests have been negative e.g. antinuclear ab, anti-smooth muscle Ab, anti-myeloperoxidase Ab5. Pathologically, AHT is verified for specimens containing lymphocyte-dominated infiltration with plasma cells, neutrophils and fibrocytes. So far, only a few case reports (n=7) with AHT have been reported, and none amongst these reports was presented together with hypothalamic obesity (HO)3,4,5.
Patients with a craniopharyngioma (CP) tumor with suprasellar growth, often suffer from post-operative hypothalamic damage which causes intractable weight gain known as hypothalamic obesity6,7,8. To predict the risk of HO include grading the hypothalamus insult with e.g. Pascual9 and Sainte-Rose C10 and more recently with the hypothalamus (HT) volume measurements7. In instances with HT insult, there is an immediate experience of hyperphagia resulting in a very rapid weight gain, which within a few weeks to months may double the weight of the patient6. This immediate effect levels off once the patients enter a more chronic phase of HO11. In addition, patients with HO also suffer from a reduced basal metabolic rate (BMR)12. So far, caloric reduction and pharmacological intervention has had limited effect on weight13,14.
Peripherally administration of long acting incretin glucagon-like peptide 1 (GLP-1) receptor agonists (GLP-1RAs) has been shown to bind to GLP-1Rs in the brain regions involved in appetite regulation and control such as the hypothalamus and area postrema and induce weight lowing effects15,16,17. A few case reports of HO patients have presented positive results with GLP-1RAs treatment with a weight loss of about 9-22 kg18,19,20,21,22. Until the present report, the effect of a treatment therapy including both GLP-1RAs and extreme diet in patients with HO has not been studied.
The present case concerns a young female patient with AHT who after operation suffered from HO. Her HT damage could retrospectively be predicted by Magnetic Resonance Imaging (MRI) investigations based on Pascual9, Sainte-Rose C10 and Fjalldal7. Prednisone treatment together with GLP-1RAs reduced the AHT. At two occasions the patient’s weight was dramatically reduced by a very strict caloric diet, however with short endurance.
Diagnostic studies
Grading of hypothalamic involvement and measurement of hypothalamic volume
We used the suggestion of HT injury grading on MRI by Sainte-Rose10 including the following criteria: Grade 0 = no compression of HT, Grade 1 = compression of HT, Grade 2 = severe involvement of HT or unidentifiable HT (Figure I. A.). Pascual9 used the Mamillary Body Angle (MBA) as a criterion for HT involvement (Figure I. B.). The MBA, displayed by the MBs on mid-sagittal MRI, was defined as the angle formed by the intersection of a plane tangential to the base of one of the MBs with the plane tangential to the floor of the fourth ventricle Pascual9. An obtuse angel was a criterion for HT damage. In addition, hypothalamic volume measurement to grade the extent of HT damage was also used (Figure I. C.), methods shown in Gabery23.
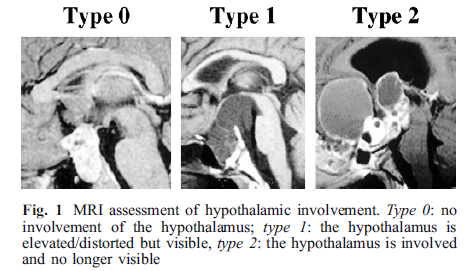
Figure 1A: Hypothalamic damage grading by Sainte-Rose10.
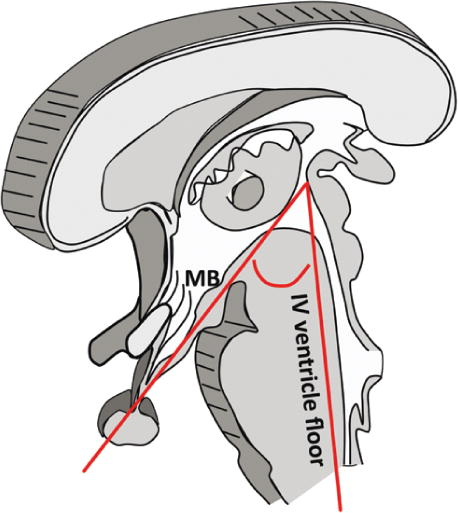
Figure 1B: Hypothalamic damage grading by Pascual9.
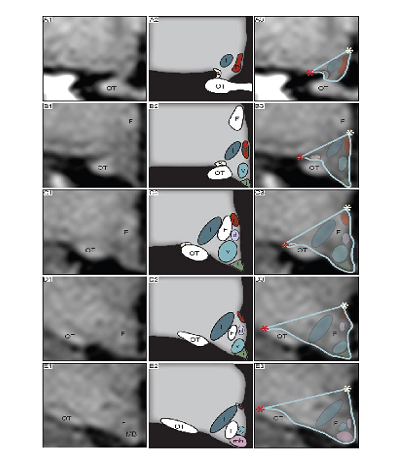
Figure 1C: Hypothalamic damage grading with volume measurement by Gabery23
Clinical case presentation with detailed MRI description presented in legends
A 19-year-old woman presented with a ten month history of visual disturbances and headache. The ophthalmological examination showed left-sided homonymous visual field deficits and a visual acuity of 0.8 in the right eye and 1.0 on the left eye. The preoperative MRI demonstrated a large contrast enhancing lesion compressing the optic chiasm (Figure II. a. b.). Preoperative baseline hormonal investigations were normal. In July 2008 she was operated for the suspicion of a CP via the zygomatio-temporal rout. The microscopic examination of the specimen revealed no typically CP cells, but inflammatory cells of mainly neutrophilic type and T-lymphocytes. Immunohistochemistry identified scattered CD3+ and CD45RO+ T lymphocytes (Figure III. a. b.). No malignant transformation was recorded. A range of tests were performed in order to identify the etiology of the inflammatory process. The results from computed tomography of thorax and abdomen were negative for lymphoma and other type os mass. Chest x-ray showed no sign of sarcoidosis or tuberculoma and blood samples were negative for serum Angiotensin converting enzymes (ACE). Tests of the cerebrospinal fluid cytology showed no malignant cells nor beta-HCG or Placental alkaline phosphatase (PLAP), but only plasma cells, granulocytes and a few lymphocytes. On post-operative ophthalmological examination, the patient presented with left-sided ptosis and mydriasis due to oculomotor nerve palsy and also a left-sided hemianopsia. Two days after operation an MRI showed a reduced inflammatory mass (Figure II. c.). Immediately postoperatively the patient developed transient syndrome of diabetes insipidus, and later adrenocortical hormone-, antidiuretic hormone- and thyroid hormone deficiencies which were pharmacologically substituted. Serum levels of estradiol, FSH and LH levels were low with amenorrhea and the patient received sex hormone substitution at later stages in the follow up.
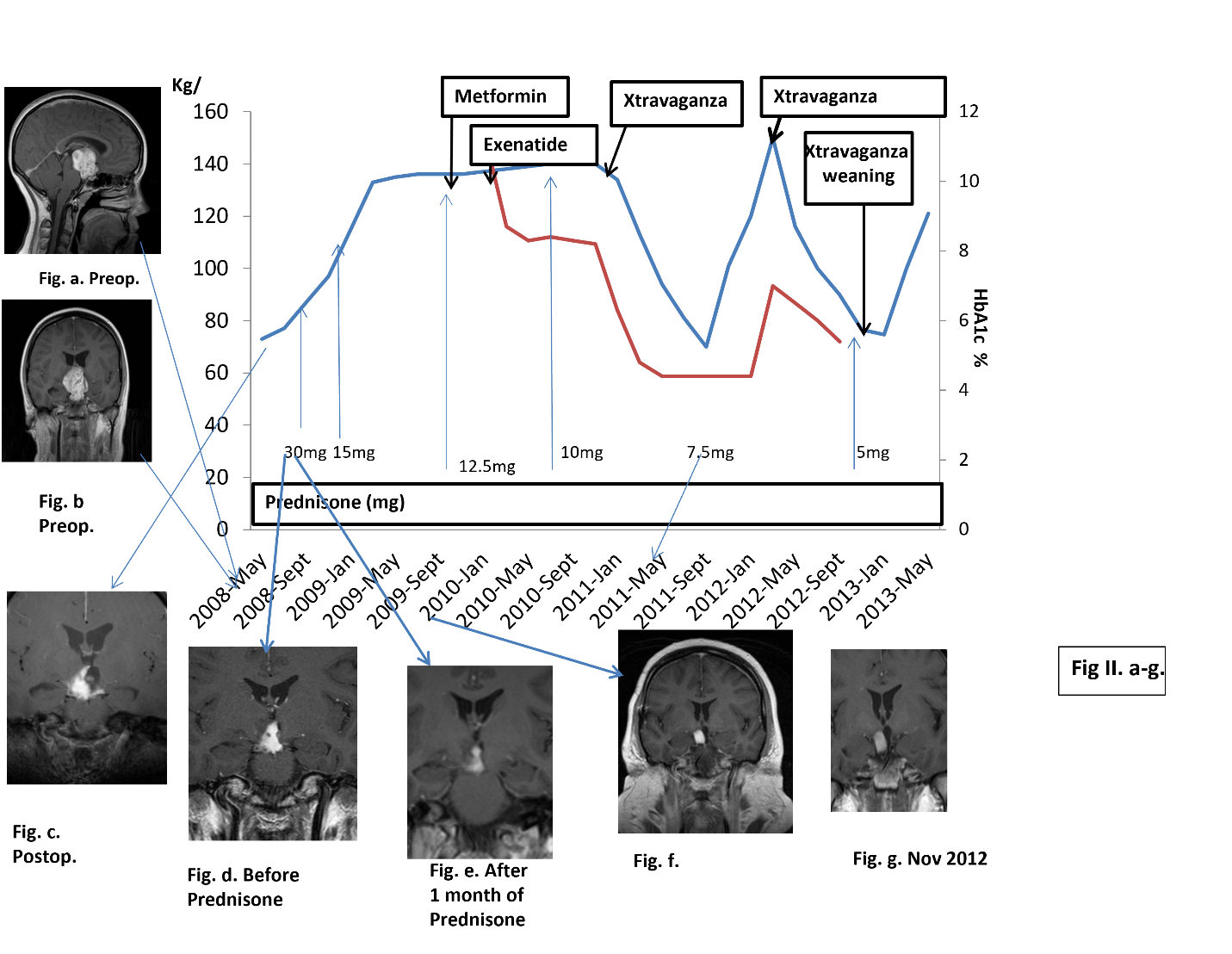
Figure II a-g: All Magnetic Resonance Imagins were T1 weighted and with iv Gadolineuem. Time table of treatment and weigth (blue line) HbAIc % (red line). Magnetic Resonance Imaging with date references
a, b. Demonstrated a contrast enhancing lesion (37x34 x42 mm) in midline with suprasellar extension compressing the optic chiasm and displacing the Mamillary Bodies (MB) and 3rd ventricle, which are unidentified, MB angle (MBA) was obtuse9 and HT grade10.
c. Two days postoperatively an MRI showed a remaining hydrocephalus. The inflammation was reduced to 16x14x21 mm and the floor of the 3rd ventricle was unidentifiable. MBA was obtuse9, Grade 1-210.
d. Just before this start of Prednisone an MRI showed an inflammatory lesion that reached the floor of the 3rd ventricle, which probably was intact. On a sagital MRI projection the posterior HT was affected but not clearly the anterior part of HT. And MBA was > 9010 with Grade 1-29.
e. One month after the start of Prednisone a reduction of the size of the contrast enhancing lesion (12x11x16 mm) was shown, which reached to the floor of the 3rd ventricle which was intact. HT was still affected. The MBA still obtuse with no change compared to October 2008.
f. In January 2010 MRI showed a slight reduction of the contrast enhancing lesion (12x10 x14 mm) and there was an improvement in vision and visual field status. On sagital MRI posterior HT was still compressed, but with less compression compared to December 2008. MBA was still obtuse10.
g. An MRI in November 2012 showed no clear change in the inflammatory process compared to January 2010, but better compared to December 2008. The floor of the 3rd ventricle was intact.
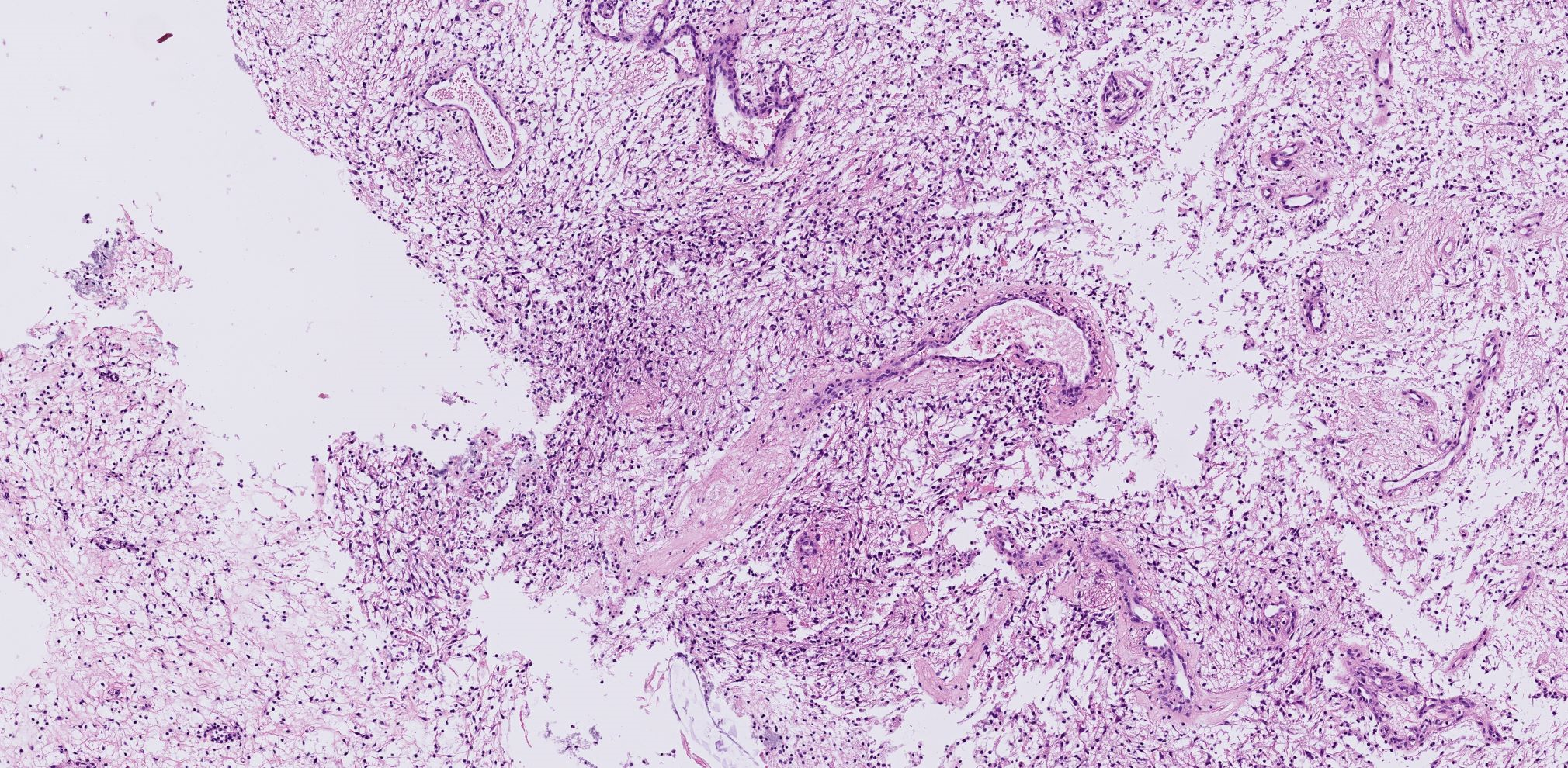
Figure III.a
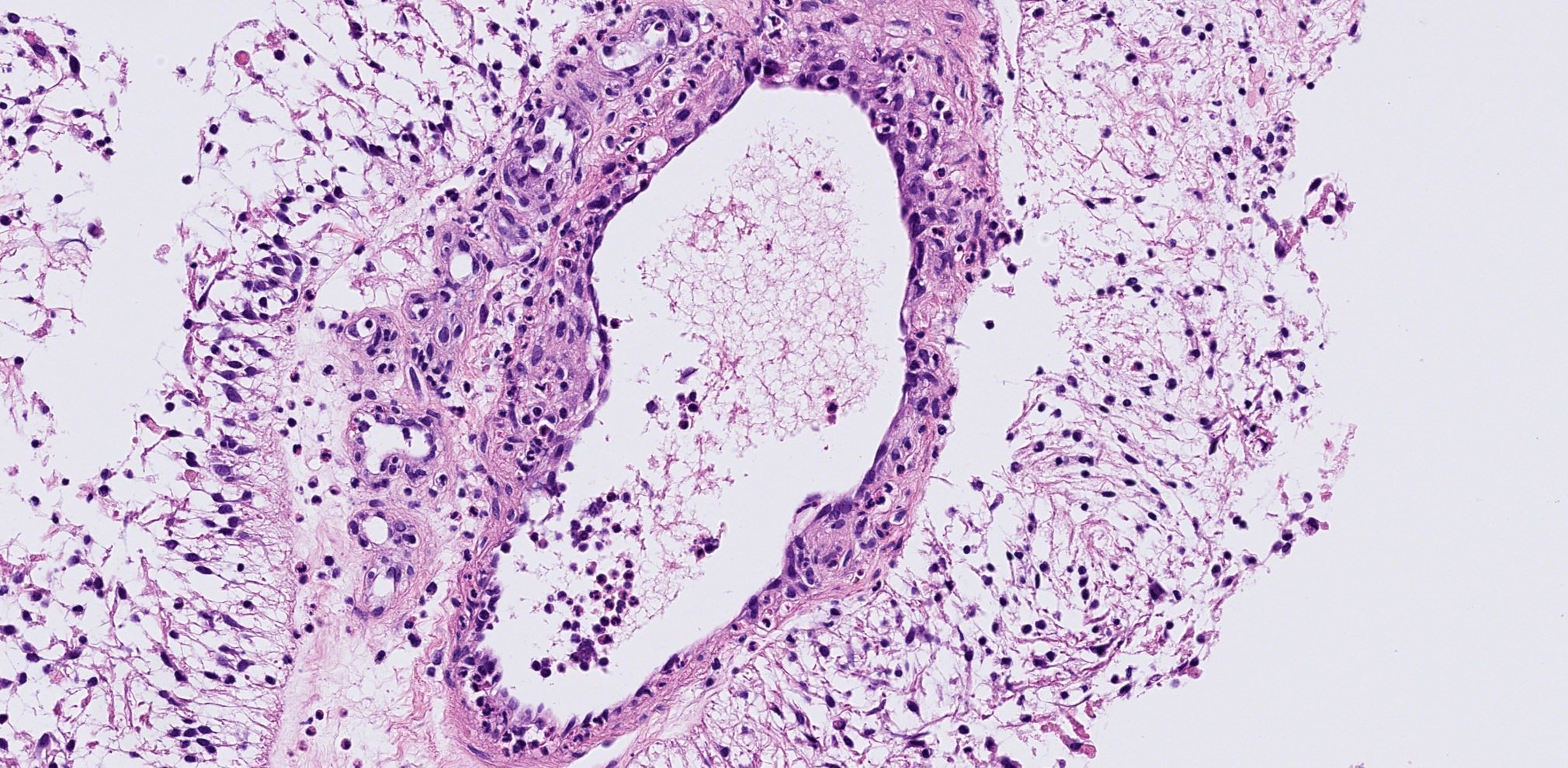
Figure III.b
Figure III. a. b. The microscopic examination of the specimen revealed no typically craniopharyngioma cells, but inflammatory cells of mainly neutrophilic type and T-lymphocytes.
a. Overview of a tissue sample with gaps in tissue with mild inflammatory/reactive tissue.
b. Detail of inflammatory cells of mainly neutrophilic type and T-lymphocytes surrounding the vessel bed. Immunohistochemistry identified scattered CD3+ and CD45RO+ T lymphocytes.
On the suspicion of an inflammatory process, the patient started at four months postoperative on 30 mg of Prednisone (Figure II. d.). A month later, MRI showed a reduction of the size of the inflammatory lesion (Figure II. e.). Ophthalmological examination showed improvement of visual fields defects. Prednisone was then gradually tapered to 15 mg over a 5-month period, and the dose was subsequently maintained for the following 10 months under MRI observation and visual examinations. In January 2010, (Figure II. f.), an MRI showed a slight reduction of the contrast enhancing lesion along with an improvement in vision and visual field status.
A year before the operation her weight was 75 kg. Six months preoperatively she started physical training which resulted in 5 kg weight reduction to 70 kg. Thus, no substantial weight gain was noted that could be related to the inflammatory lesion pre-operatively. The patient preoperative weight was 73 kg. As illustrated in Figure II., there was a rapid weight gain of 24 kg during 3 months post operation and before the initiation of high dose Prednisone therapy. After 4 months of Prednisone treatment her weight gain was 36 kg. At one year following the operation, her total weight gain was 63 kg, thus to a level of 136 kg. Two years postoperatively her weight was 140 kg (Figure II.). The total weight reduction after initiation of GLP-1RA was about -5 kg.
In addition to the combined treatment with Metformin, GLP-1RA in an increasing dose to 1.8 mg and Prednisone lowered to 10 mg, she started in January 2011 a low calorie diet program (Xtravaganza i.e. 500 kcal/day). This resulted in an altogether beneficial effect on weight reduction (15 kg in 1 month), glycemic index (HbA1c -1.5% in 8 weeks, from 6.3% to 4.8%). She lost 10 kg in the next 5 weeks (to 108 kg) and an additional 21 kg during the following 12 weeks (to 87 kg) (Figure II.). Thus, her weight loss started on the third year after operation and during this year her total weight reduction was 61 kg.
At her follow-up visit in February 2010 (Figure II.), blood tests showed HbA1c 11.4 % and she started taking Metformin 500 mg. At this point the Prednisone dose was reduced (12.5 mg). To control her increasing weight and blood glucose values, the patient received GLP-RA, exenatide 5 µg sc daily. HbA1c prior to DM treatment was 11.4% which decreased to 8.7% after one month of the combined treatment. The exenatide dose was in May 2010 increased to 10 µg, with slight improvement of HbA1c (8.3%) and 3 months later the Prednisone was reduced to 10 mg (Figure II.) which was followed by a weight reduction (-2 kg). In November 2010 exenatide was switched to liraglutide on weekly bases, to an increasing dose. In May 2011 the Prednisone dose was further reduced to 7.5 mg (Figure II.). Vision was normal but visual field examination unchanged since June 2010 with a left homonymous hemianopia. On follow up visit in September 2011 treatment with GLP-1RA was stopped, Metformin dose reduced, and Prednisone dose unchanged (7.5 mg) and her HbA1C was within normal range. After 8 months she discontinued Xtravaganza (Figure II.) and tried a healthy ordinary food diet, which directly resulted in weight gain (20 kg during 1 month). During that time, she had started studying again which made it difficult to confine to her strict routine pertaining to her eating habits. In March 2012 she weighed 150 kg (HbA1C 7%) and started again Xtravaganza for 8 months. Again, with positive results and she reaches her preoperative weight of 70 kg (Figure II.). In Nov 2012, her weight was at 76 kg, the patient began weaning off Xtravaganza and gradually increases her caloric intake. This resulted in an almost instantly weight gain, accompanied with loss of implemented routines. In the early phase of weaning, Prednisone was decreased to 5 mg. In January 2013, the patient underwent acute cholecystectomy by the laparoscopic route. In February 2013, she starts gaining weight and by April 2013 the patients’ weight had increased to 121 kg in spite of continued “Cognitive Behavioral Therapy”. In June 2013, she had lost control of her eating and suffered from low energy and slight memory problems. An MRI obtained in October 2013 (Figure IV. h.) revealed an anterior HT with clearly less compression but unchanged inflammation since previous MRI (Figure II. g.). In January 2014 her weight was about 150 kg, and she had no interest in starting Xtravaganza again. At this point her medication comprised of, tablets of Thyroxine, DDAVP (Minirin) 60 ug once daily at night, oral contraceptive, Prednisone 7.5 mg and Metformin.
MRI performed in April 2015, showed an unchanged suprasellar mass approaching the bottom of the hypothalamus, thus, unchanged compared previous MRI (Figure IV. h. i.). Hypothalamic volume performed on the MRI, estimated a hypothalamic volume of 63 mm3 on the right side, and 22 mm3 on the left side, resulting in a total hypothalamic volume of 85 mm3. In comparison to a previous study including control women of the same age as the patient (n=18) the median total HT volume was 870 mm3 (range 775- 941)23 (Figure V. A-F). The latest MRI in May 2021 showed an unchanged contrast enhancing suprasellar mass and hypothalamic volume, compared to March 2019 and February 2018.
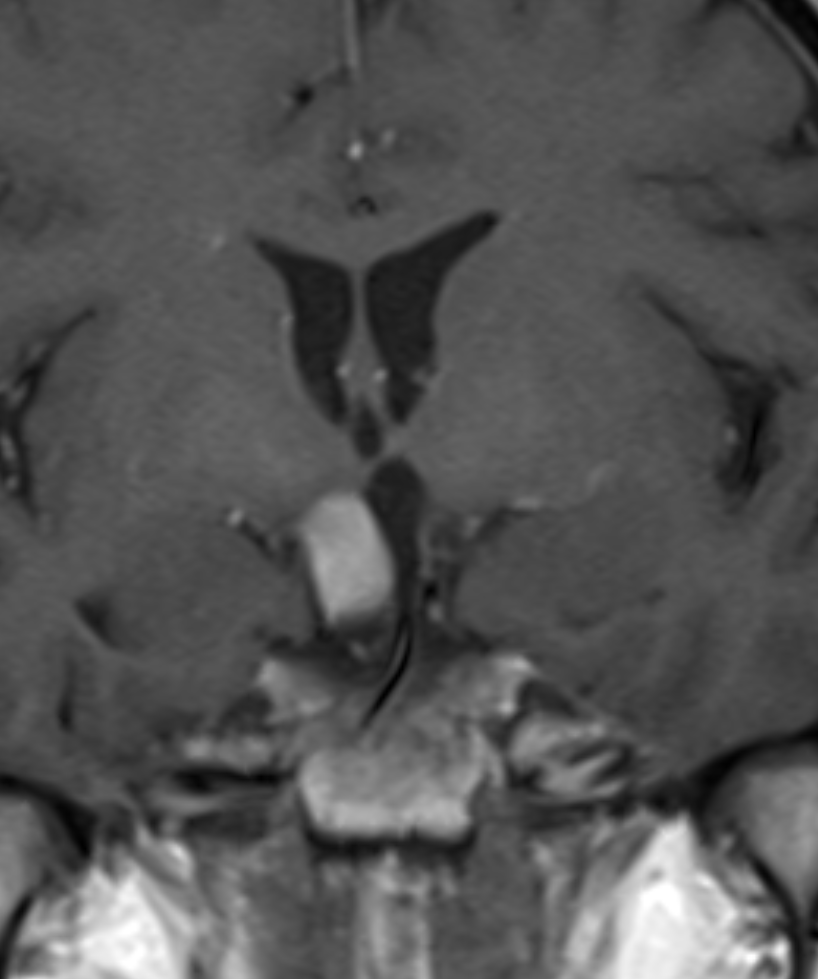
Figure IV. h
Figure IV. i
Figure IV. h. i. In October 2013 an MRI showed an anterior part of the HT with some fluid but posterior HT seemed now unaffected. The size of the inflammation was 11x15x10 mm, thus, almost the same as the previous MRI. Grade 0-110 and MBA was about 90° 9.
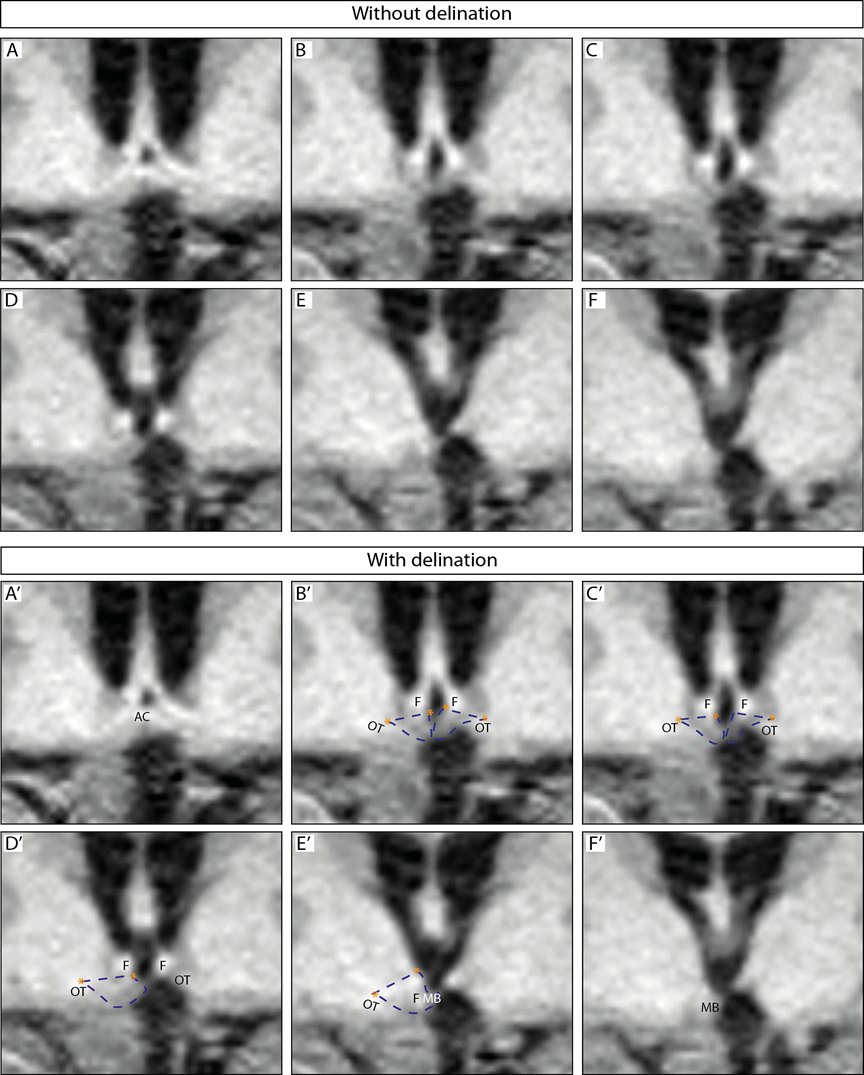
Figure V. A-F. Hypothalamic measurements by Gabery23
The images show an overview of the hypothalamic region. A-F represents the hypothalamic region on a coronal plane, from a rostral to caudal direction; A’- F’ represents the same region with dashed blue lines depicting how the hypothalamus was delineated. Landmarks such as the hypothalamic sulcus and the lateral or medial edge of the optical tract are represented by orange stars. Abbreviations: anterior commissure, AC; fornix, F; optical tract, OT; mammillary body, MB. The hypothalamic region appeared shifted upwards from the floor of 3rd ventricle to the ventricles beginning from the medial hypothalamus from the infundibulum and continuing in a caudal-rostral direction towards the anterior part of the hypothalamus The infundibular region was visible although slightly distorted. The left side appeared to lack portions of the medial hypothalamic region (Fig. V. D, E, D’ E’) and was thus present on fewer slides than the right hypothalamic region. The right side appeared to be intact and not lacking any portion of the hypothalamus, however shifted upwards toward the ventricle (Fig. V. A and A’ to F and F’). The posterior portion of the hypothalamus at the level of the MB appeared to be intact for both hemispheres however also shifted upwards toward the ventricle.
In April 2015 when HT volume measurement was performed, the patient weight was 156 kg, with an BMI 55, and DEXA revealed a fat mass 61% (93 kg), lean mass 61 kg and with normal bone mineral content. Fasting levels of serum ghrelin was 441 ng/L (reference interval 550-2130 ng/L) and leptin 208 µg/L (reference interval < 15 µg/L). Total cholesterol 4.8 (reference interval 3.3-6.9), low density lipoprotein 2.9 (reference interval (≤30 years: 1.2–4.3 mmol/L), high density lipoprotein 1.0 (reference interval females 1.0–2.7 mmol/L), triglyceride 3.0 (reference interval 0.4–2.6 mmol/L) and fT4 16 nmol/l, reference interval T4 (12–22 pmol/L) and free T3 5.5 nmol/l (reference interval 3.6–6.3 pmol/L). The patient was treated with oral Metformin 500 mg twice daily, Minirin 60 ug at night, Thyroxine 150 ug daily, Prednisone 7.5 mg daily, Alendronate 70 mg daily. In November 2018 the patient stopped her Prednisone treatment.
In September 2020, an MRI showed no change in appearance compared to MRI February 2018. Her weight was 147 kg, and she was supplemented with the same Tablets as previously; with an addition of Hydrocortisone 10 mg twice daily, Novofem once daily, (combination of Estradiol and Noretisterone). The most current MRI obtained in May 2021, revealed again no change in appearance neither of the HT nor of the status of the inflammation.
The patient’s own experience of hypothalamic obesity
The patient described how she lost the feeling of satiety directly after the operation and she had to restrain her eating habits with alternative means. The patient’s family members also describe an immediate increase in the patient’s appetite, postoperatively. She was constantly thirsty, drinking larger quantities of drinks high in carbohydrates. The patient describes a form of psychological craving for food which she differentiates from hunger sensation. With time, the patient had adjusted to a routine of regular meals with fixed meal sizes which she had adjusted in order to avoid an extreme sense of hunger. According to the patient a very strict daily routine is the most important factor when it comes to maintaining weight loss. Once this regime is interrupted, the patient found herself in the extremes of overwhelming hunger or fullness.
Discussion
We present a unique case of AHT diagnosed by transcranial operation. The specimen revealed inflammatory cells of mainly neutrophilic type and T-lymphocytes. Immunohistochemistry identified scattered CD3+ and CD45RO+ T lymphocytes. Malignant transformation or other causes were excluded by extensive investigations including liquor analyses. The MRI seems to be of diagnostic importance in AHT4. On T1-weighted imaging the AHT is characteristically hypo-or iso-intense, compared with normal white matter, and on T2-weihgted imaging the mass was homogenous and significantly enhanced by contrast. By applying Prednisone treatment during the following years, a further reduction of the inflammation was accomplished together with normalization of vision and improvement of visual fields. When no further reduction of the inflammatory mass was recorded the Prednisone treatment was terminated.
The patient suffered from immediate severe hypothalamic damage after the operation, resulting in HO. Grading according to Pascual9 and Saint Rose10 revealed that the posterior HT on MRI was severely affected and remained displaced during follow up. In addition, after seven years of follow up the volume of the HT was 1/10 of normal (normal 775-941 mm3)24,7. A HT volume of 83 mm3 is in the range where previously studied CPs all suffered from HO7. With the introduction of GLP-1RAs along with very strict dietary restriction the patient was twice able to return to her previous weight, but with short endurance. MRI performed in March 2019, 11 years post-operation, showed a distorted and significantly reduced hypothalamic volume. The contrast enhancing lesion remained unchanged for 4 years and thus Prednisone was postponed. The patient remained severely obese until end of this follow up.
Pathogenesis of hypothalamic obesity
The severely damaged HT start an immediate pathogenesis of obesity, with increased energy intake beyond what is required. A variety of mechanisms have been reported for this condition25,26. Firstly, decreased sympathetic nervous system tone27 leads to reduced physical activity and energy expenditure but also to a reduction in lipolysis favouring adiposity28,12. Secondly, increased vagal tone leads to increased pancreatic insulin secretion13,29, with increased adipocyte insulin sensitivity30 promoting energy storage in adipocytes and in the liver31. Thirdly, damage to neurons in ventromedial hypothalamus (VMH), result in leptin resistance12 which causes a manifestation of a starvation response, illustrated by an increased leptin level (208 ug/L) in the present case. These processes collectively result in a vicious circle of a constant weight gain.
Uncontrolled weight gain with lack of postprandial satiety, typical for the HO syndrome, was illustrated in the present case by the rapid weight gain of 24 kg during 3 months directly after operation and before the initiation of the high dose steroid therapy (Figure II.). After the initiation of 30 mg Prednisone her weight accelerated to 130 kg in spite of a reduction of inflammation on the MRI after one month (Figure II. e.). The typical lack of postprandial satiety seems to last about 6 months but even until 24 months before it levels off11. The patient’s weight stabilized at a very high level after about one year after operation.
The effect of GLP-1RAs on weight
The patient was commenced on GLP-1RA, exenatide, at the same time as tapering of Prednisone had started which makes it difficult to evaluate the effect of reduced steroid dose or exenatide on weight loss. GLP-1RAs has been reported to down regulate pro-inflammatory responses at the cellular and molecular level in patients with diabetes mellitus, but in the present situation this contribution should be considered minor in the context of Prednisone treatment32. Nevertheless, GLP-1RAs facilitate weight loss, by a lowering of gastric emptying, and also increasing satiety at the HT level15,16,17.
The effect of caloric restriction
The extreme diet introduction with only 500 kcal/day together with Metformin and then later another GLP-1RA, resulted to an altogether beneficial effect on weight reduction, better glycemic index, and the patient’s wellbeing (Figure II.). This was somewhat surprising considering that our previous studies have shown that CP patients with HO have lower energy intake compared to healthy controls which does not contribute to weight reduction12. It is possible that the post-operative lack of postprandial satiety now is reduced, 30 months later, facilitating a weight reduction. However, the present diet attempt was in fact a kind of starvation.
Weight loss as a result of lower calorie intake reduces energy expenditure by lowering basal metabolic rate (BMR) as well as thyroid hormones and catecholamine concentrations making further weight loss to be difficult and rather predisposes to weight regain33. A low BMR is thought to be caused by relative leptin resistance resulting from restricted diet34. From “The Biggest looser” studies it was shown that massive weight loss resulted in persistent metabolic rate slowing causing a reduced BMR which can last up to six years35. After six years and despite substantial weight regain in this population, the BMR remained at 500 kcal/day thus lower than expected35.
Compared to the overall weight loss presented in other case reports of HO with GLP-1RAs in the literature (range 9-22 kg)18,19,20,21,22 one can assume that a significant part of the weight loss in the present case (Figure II.) was attributed to the extreme diet and to a much lesser part from the anti-inflammatory effect of the steroids on the HT. The importance of the diet was illustrated by the fact that twice the patient’s weight increased dramatically back to her previous level of 150 kg as soon as the diet discontinued. Her weight was stable on this level during the following years when the Prednisone dose was discontinued due to no further reduction in the inflammation.
This case report illustrates that attenuation of the inflammation with Prednisone is possible in AHT as well as an improvement of vision. The regression of the inflammation on much reduced HT volume had however, hardly any effect on weight loss. Although being speculative there is a possibility that a GLP-1RAs might also attenuate the inflammatory process36. It shows also that in HO, extreme diet is successful in reducing weight in combination with a GLP-1RAs. However, it also shows brutally how difficult it is to maintain a weight reduction after such a rapid and extreme diet introduction. With the growing knowledge of the relationship between hypothalamic damage and energy expenditure new therapeutic options that modulate hypothalamic function is needed to battle against obesity.
Conflict of Interest
All authors have nothing to disclose.
Funding
This work was supported by the Swedish Children's Cancer Foundation, and the Medical Faculty, Lund University, Sweden.
References
- Caturegli P, Newschaffer C, Olivi A, et al. Endocrine Reviews. 2005; 26(5): 599-614.
- Wei Q, Yang G, Lue Z, et al. Clinical aspects of autoimmune hypothalamitis, a variant of autoimmune hypophysitis: Experience from one center. J Int Med Res. 2020; 48(3): 1-13.
- Wang XL, Lu JM, Yang LJ, et al. A case of relapsed autoimmune hypothalamitis successfully treated with methylprednisolone and azathioprine. Neuro Endocrinol Lett. 2008; 29: 874-876.
- Zhang S, Ye H, Zhang Z, et al. Successful diagnosis of hypothalamitis using stereotactic biopsy and treatment: A case report. Medicine (Baltimore). 2015; 94: e447.
- Bertulli L, Bertani GA, Gianelli U, et al. Long-standing isolated autoimmune hypothalamitis diagnosed with endoscopic transventricular biopsy. World Neurosurg. 2017; 105: e1035-1036.
- Muller HL. Childhood craniopharyngioma. Recent advances in diagnosis, treatment and follow-up. Horm Res. 2008; 69: 193-202.
- Fjalldal S, Follin C, Gabery S, et al. Detailed assessment of hypothalamic damage in craniopharyngioma patients with obesity. Int J Obes (Lond). 2019; 43(3): 533-544.
- Poretti A, Grotzer MA, Ribi K, et al. Outcome of craniopharyngioma in children: long-term complications and quality of life. Dev Med Child Neurol. 2004; 46(4): 220-9.
- Pascual JM, Prieto R, Carrasco R, et al. Displacement of mammillary bodies by craniopharyngiomas involving the third ventricle: surgical-MRI correlation and use in topographical diagnosis. J Neurosurg. 2013; 119(2): 381-405.
- Sainte-Rose C, Puget S, Wray A, et al. Craniopharyngioma: the pendulum of surgical management. Childs Nerv Syst. 2005; 21(8-9): 691-5.
- Sterkenburg AS, Hoffmann A, Gebhardt U, et al. Childhood craniopharyngioma with hypothalamic obesity - no long-term weight reduction due to rehabilitation programs]. Klin Padiatr. 2014; 226(6-7): 344-50.
- Holmer H Pozarek G, Wirfält E, et al. Reduced energy expenditure and impaired feeding-related signals but not high energy intake reinforces hypothalamic obesity in adults with childhood onset craniopharyngioma. J Clin Endocrinol Metab. 2010; 95(12): 5395-402.
- Lustig RH. Autonomic dysfunction of the β-cell and the pathogenesis of obesity. Rev Endocr Metab Disord. 2003; 4: 23-32.
- Danielsson P, Janson A, Norgren S, et al. Impact Sibutramine therapy in children with hypothalamic obesity or obesity with aggravating syndromes. J Clin Endocrinol Metab. 2007; 92: 4101-4106.
- Gabery S, Salinas CG, Paulsen SJ, et al. Semaglutide lowers body weight in rodents via distributed neural pathways. JCI Insight. 2020; 5(6): e133429.
- Kanoski SE, Hayes MR, Skibicka KP. GLP-1 and weight loss: unraveling the diverse neural circuitry. Am J Physiol Regul Integr Comp Physiol. 2016; 310(10): R885-95.
- Secher A, Jelsing J, Baquero AF, et al. The arcuate nucleus mediates GLP-1 receptor agonist liraglutide-dependent weight loss. J Clin Invest. 2014; 124(10): 4473-88.
- Simmons JH, Shoemaker AH, Roth CL. Treatment with Glucagon-peptide-like-1 agonist Exendin-4 in a patient with hypothalamic obesity Secondary to intracranial tumor. Hom Res Paediatr. 2012; 78: 54-58.
- Thondam SK, Cuthbertson DJ, Aditya BS, et al. A glucagon-like peptide-1 (GLP-1) receptor agonist in the treatment for hypothalamic obesity complicated by type 2 diabetes mellitus. Clin Endocrinol (oxf). 2012; 77(4): 635-7.
- Zoicas F, Droste M, Mayr B, et al. GLP-1 analogues as a new treatment option for hypothalamic obesity in adults: report of nine cases. Eur J Endocrinol. 2013; 168(5): 699-706.
- Astrup A, Carraro R, Finer N, et al. NN8022-1807 Investigators. Safety, tolerability and sustained weight loss over 2 years with the once-daily human GLP-1 analog, liraglutide. Int J Obes (Lond). 2012; 36(6): 843-54.
- Roth CL, Perez FA, Whitlock KB, et al. A phase 3 randomized clinical trial using a once-weekly glucagon-like peptide-1 receptor agonist in adolescents and young adults with hypothalamic obesity. Diabetes Obes Metab. 2021; 23(2): 363-373.
- Gabery S, Georgiou-Karistianis N, Lundh SH, et al. Volumetric analysis of the hypothalamus in Huntington Disease using 3T MRI: the IMAGE-HD Study. PLoS One. 2015; 10(2): e0117593.
- Fjalldal S, Rylander L, van Westen D, et al. Brain white matter lesions are associated with reduced hypothalamic volume and cranial radiotherapy in childhood-onset craniopharyngioma. Clin Endocrinol (Oxf). 2021; 94(1): 48-57.
- Schwartz M, Woods S C, Porte Jr D, et al. Central nervous system control of food intake. Nature. 2000; 404: 661-71.
- Spiegelman BM, Flier JS. Obesity and the regulation of energy balance. 2001; 104(4): 531-43.
- Schofl C, Schleth A, Berger D, et al. Sympathoadrenal counterregulation in patients with hypothalamic craniopharyngioma. J Clin Endocrinol Metab. 2002; 87: 624-629.
- Harz KJ, Hermann L Müller, Edith Waldeck, et al. Obesity in patients with craniopharyngioma: assessment of food intake and movement counts indicating physical activity. J Clin Endocrinol Metab. 2003; 88: 5227-5231.
- Bray GA, Gallagher TF. Manifestations of hypothalamic obesity in man: a comprehensive investigation of eight patients and a review of literature. Medicine (Baltimore). 1975; 54: 301-333.
- Kreier F, Fliers E, Voshol PJ, et al. Selective parasympathetic innervation of subcutaneous and intra-abdominal fat-functional implications. J Clin Invest. 2002; 110: 1234-1250.
- Bray GA, Inoue S, Nishizawa Y. Hypothalamic obesity: The autonomic hypothesis and the lateral hypothalamus. Diabetologia. 1981; 20(Suppl 1): 366-377.
- Chaudhuri A, Ghanim H, Vora M, et al. Exenatide exerts a potent anti-inflammatory effect. J Clin Endocrinol Metab. 2012; 97(1): 198-207.
- van Iersel L, Brokke KE, Adan RAH, et al. Pathophysiology and Individualized Treatment of Hypothalamic Obesity Following Craniopharyngioma and Other Suprasellar Tumors: A Systematic Review. Endocrine reviews. 2019; 40(1): 193-235.
- Lecoultre V, Ravussin E, Redman LM. The fall in leptin concentration is a major determinant of the metabolic adaptation induced by caloric restriction independently of the changes in leptin circadian rhythms. J Clin Endocrinol Metab. 2011; 96(9): E1512-6.
- Fothergill E, Guo J, Howard L, et al. Persistent metabolic adaptation 6 years after "The Biggest Loser" competition. Obesity (Silver Spring). 2016; 24(8): 1612-9.
- Shan Y, Tan S, Lin Y, et al. The glucagon-like peptide 1 receptor agonist reduces inflammation and blood-brain barrier breakdown in an astrocyte-dependent manner in experimental stroke. J Neuroinflammation. 2019; 16(1): 242.
