Commentary: Emerging role of GIP and related gut hormones in fertility and PCOS
Dawood Khan, R Charlotte Moffett*
SAAD Centre for Pharmacy and Diabetes, Ulster University, Coleraine, Northern Ireland, UK
Abstract
Polycystic ovary syndrome (PCOS) is a common endocrine disorder associated with infertility which affects one in ten women in the United Kingdom. Women with PCOS are typified by insulin resistance, gestational diabetes and obesity. Therefore, a close association between reproductive function and nutrition is postulated. However, regulatory pathways common to energy and reproductive function have received little attention. Recent research shows rapid amelioration of infertility, PCOS and type 2 diabetes following Roux-en-Y bariatric surgery (RYGB). This occurs prior to weight loss suggesting involvement of gut derived factors. Therefore, gut hormones emerge as key players in the regulation of both energy homeostasis and possibly reproductive function. Alteration of gut peptide levels including GLP-1, GIP, PYY, ghrelin, NPY and neurotensin post-bariatric surgery suggest a plausible mechanism behind beneficial effects of RYGB. Furthermore, expression of gut peptide receptors within the reproductive axis strengthen the idea of involvement of these hormones in the remission of fertility post-surgery. The present commentary discusses the role of these important gut peptides and their receptors in the regulation of female reproductive system in the light of a recent article published by our laboratory. Understanding the functional relationship between the gut and reproductive axis will help us to identify novel and less invasive alternatives to bariatric surgeries for reproductive and related metabolic disorders.
Substantial evidence reveals a correlation between female infertility and type 2 diabetes (T2D). Major cause of female infertility associated with metabolic syndrome such as T2D is polycystic ovarian syndrome (PCOS)1. PCOS, a reproductive and metabolic disorder affecting 5–10% of women in their fertile years, which induces symptoms including reproductive abnormalities, altered androgen levels, hyperinsulinaemia together with insulin resistance and risk of gestational diabetes2. Roux-en-Y bariatric surgery (RYGB) is a gold standard treatment for long-term weight loss in morbidly obese people. In addition to weight loss, RYGB promotes blood glucose control, corrects menstrual disturbances and ameliorates PCOS thereby increasing fertility3,4,5,6. Interestingly, alteration of gut hormones such as gastric inhibitory polypeptide (GIP), glucagon-like peptide-1 (GLP-1) and peptideYY (PYY) has been shown to play key roles in the remission of obesity and diabetes post bariatric surgery7. However, the functional connection between gut hormones and reproductive dysfunction has received little attention.
In the recent work published by our laboratory, Moffett and Naughton (2020)8 highlight metabolic benefits of RYGB in the treatment of PCOS and possible mechanisms through which gut hormones may be involved in female reproductive function. In recent years, gastric bypass surgeries have become an effective tool to treat obesity and improve related comorbidities such as insulin resistance, cancer, reproductive dysfunction and cardiovascular disorders are observed post-surgery. However, high-cost, invasiveness and irreversibility of the procedure prevents RYBG becoming a more common and popular mode of treatment. Therefore, unravelling the mechanism(s) behind multifaceted benefits of bariatric surgeries is vital to provide less invasive and cost-effective alternatives targeting tissue-specific impacts.
Interestingly, even before weight loss is achieved, RYGB confers other disproportionate health benefits9. Although the exact mechanism is unclear, the Moffett & Naughton (2020)8 article gives important clues. Firstly, increased secretion and circulation of key gut peptides including GLP-1, PYY and oxyntomodulin is observed which may be a vital driving factor post RYGB10,11,12. These hormones act as agonists for GLP-1R, NPYRs and glucagon receptors which modulate various pathways regulating satiety, beta-cell function and fat metabolism. Secondly, following RYGB, gut hormones including GIP and ghrelin are markedly decreased in circulation. Although there are debates surrounding serum GIP levels post RYGB, role of GIP in energy storage is well studied13. Similarly, decreased levels of ghrelin post RYGB may explain reduced hunger as it stimulates appetite in short-term and in long-term plays a key role in fat storage14. In addition to the afore mentioned peptides, neurotensin is another gut hormone that has received relatively little attention with regards to bariatric surgeries. Recent clinical studies show elevated levels of pro-neurotensin postprandially after RYGB suggesting relevance of gut peptides other than incretins mediating beneficial effects of RYGB15,16,17.
An important question raised by the article is “What happens to PCOS after RYGB surgery and are same mechanisms revolving around gut hormones involved? Recent studies show significant resolution of PCOS post bariatric surgery in 96 % of affected women8. Studies involving 2130 females with mean BMI of 48.1 kg/m2 significantly reduced incidence of PCOS from 45.6% to 6.8% at 12-month post-operative follow up18. This included positives effects on hirsutism and menstrual irregularities which are key clinical symptomatology in PCOS18. In 1960s, close association between critical body weight and female reproductive capacity was postulated suggesting adaptive response to poor nutrition19.
Although not being part of the hypothalamo-pituitary-gonadal reproductive axis, gut peptides may affect endometrial and other related functions. These gut peptides interfere with the regulation of reproductive systems at various levels based on sex, species or stage of puberty20. One such peptide is GLP-1. GLP-1, mainly secreted from intestinal L-cells is evidently shown to be involved in modulation of hypothalamic gonadotropin-releasing hormone (GnRH) neurons20,21,22,23 (Figure1). Interestingly, an increased level of plasma GLP-1 is also observed during pro-oestrous phase in time-related manner24. Furthermore, expression of GLP-1 receptor mRNA in the hypothalamus peaked during pro-oestrous stage suggesting a crosstalk between GLP-1 and its receptor22. Studies with GLP-1R knockout mice revealed significantly decreased number of ovarian follicles25. Data from these studies suggest GLP-1 as a key player in fertility.
Another important incretin hormone highlighted in the review article by Moffett & Naughton (2020)8 is GIP. GIP, produced by intestinal K cells, increases glucose uptake and inhibition of lipolysis in adipocytes after inhibition of 11β-hydroxysteroid dehydrogenase type 1 (11β-HSD1), an enzyme which regulates cortisol26. Furthermore, studies show decreased hepatic 11β-HSD1 activity in obese women with PCOS and expression of GIP receptors in ovaries and testes27. Women with PCOS also show increase in total GIP concentrations28. Taken together, these data propose unsuspected role of GIP as an important contributing factor in reproductive function. Evidence suggest that modulation of both GIPR and GLP-1R significantly suppresses progesterone synthesis in the presence of FSH and expression of many progesterogenic factors and enzymes29(Figure1). Further studies exploring synergistic effects of GLP-1 and GIP using dual receptor agonist/antagonist will be necessary to fully understand the significance of GLP-1R and GIPR signalling for activation of various pathways involved in female fertility.
PYY, mainly secreted from intestinal L-cells together with GLP-1 regulates feeding and beta cell function inhibiting insulin secretion30. Although, its role in reproductive axis is not well studied, it is important to note that PYY administration inhibits GnRH secretion in male rats (Figure1) and delayed the estradiol-induced LH surge in ovariectomized ewes31,32. Neuropeptide Y, another orexigenic Y receptor agonist, exhibits decreased plasma levels in overweight and obese patients with PCOS33. Interestingly, NPY response to ghrelin in obese women with PCOS was significantly reduced in plasma as shown by Romualdi et al. in 200834. As insulin inhibits hypothalamic NPY gene expression, variation in plasma NPY levels in PCOS may be attributed to insulin action33. Since NPY receptor subtypes are expressed in adrenal cortex, ovaries and pituitary35,36. It is imperative to clarify the exact role of PYY and NPY on the reproductive axis in relation to PCOS associated with obesity.
Ghrelin is a 28 amino acid peptide mainly secreted by stomach37. Ghrelin regulates glucose metabolism, food intake, motility of gastrointestinal tract and controls reproductive functions binding to growth hormone receptors. Women with obesity and PCOS showed significant reduction in plasma ghrelin levels38. Expression of ghrelin and its receptor in reproductive organs including endometrium and testis, phase-dependant ghrelin mRNA fluctuation in rat ovaries, and ghrelin mediated control of prolactin secretion in both rats and humans suggests possible role in the reproductive function39,40.
Neurotensin, a 13 amino acids neuropeptide is found in the central nervous system, small intestine and stomach41. It functions as a modulator of the dopaminergic system42.Neurotensin immunoreactivity is reported in rodent uterus, endometrium epithelium and oviduct with neurotensin suggested as a key factor in facilitating sperm function during fertilization43(Figure1). Studies are required to determine if neurotensin and its receptors mediate signalling pathways in reproduction. Figure1 below summarizes key gut hormones, predominant site of secretion, circulatory changes in RYGB and reproductive actions.
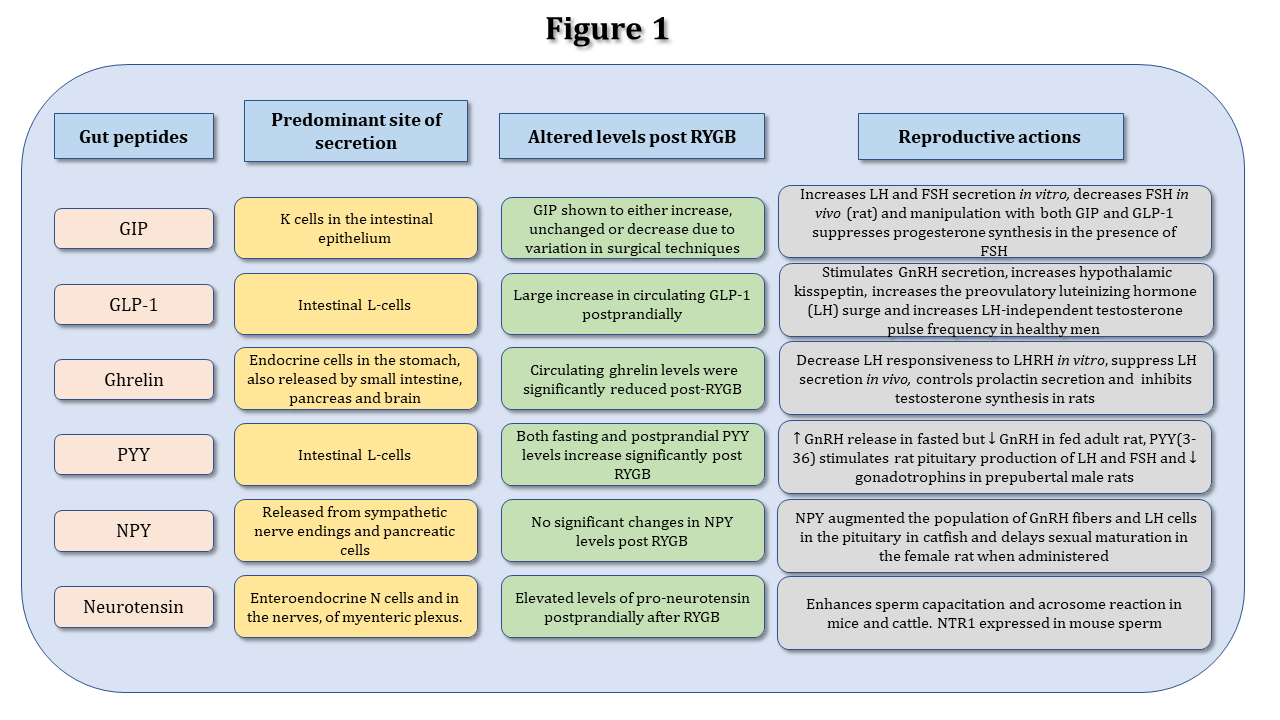
Figure 1: Summary of major gut peptides, their predominant secretion sites, adaptation post-RYGB and associated reproductive functions.
Gut hormones act to exert a variety of physiological roles mainly associated with energy and glucose metabolism. The figure summarizes the overall effect of gut hormones included in the commentary on the reproductive axis established by currently available literature.
Although obesity and PCOS frequently coexist in female adult and adolescent population, clinical studies covering the three conditions (morbid obesity, PCOS, and RYGB) simultaneously are scarcely available44,45,46. Previous study showed significant weight loss post bariatric surgery leading to complete resolution of PCOS including improvements in menstrual cycle, hirsutism and insulin resistance in 17 women47. Study by Turkmen and colleagues showed improvement in eating behaviour and clinical symptoms of PCOS post-RYGB in 9 female patients48. Similarly, all female participants with infertility and PCOS (N=6; N=5) were able to conceive post-RYGB49,50. However, one attribute common to the afore mentioned studies is small sample size limiting the interpretation of data. Although preclinical studies highlight the efficacy of RYGB in the treatment of PCOS associated with obesity, more extensive assessment is required to establish their translational potential to clinical settings. Nevertheless, studies with substantial sample size and randomized controls focusing on altered incretin and androgens levels post-RYGB in obese females may offer better understanding in this relatively new area of research.
In summary, the review by Moffett and Naughton (2020)8 opens up a fascinating field of research by highlighting the role of altered gut hormonal milieu post RYGB in ameliorating PCOS and increasing female fertility. Clearly, advantages of incretins and other related gut peptides go far beyond managing T2D and their full potential remains to be proven that may benefit patients with reproductive dysfunction. Their review should be viewed, not as an endorsement of use of incretin-based therapies in the treatment of obesity-related PCOS, but rather, as an invitation for further studies to elucidate the mechanism linking modulation of gut hormone receptors in reproductive and hypothalamic-pituitary-adrenal axis. This could reveal novel therapies for reproductive dysfunction associated with metabolic disturbances.
Acknowledgements
RCM and DK are supported by an RD Lawrence Research Fellowship awarded by Diabetes UK to RCM.
Conflict of interest
No potential conflict of interests relevant to this article were reported.
References
- Sanchez-Garrido MA, Tena-Sempere M. Metabolic dysfunction in polycystic ovary syndrome: Pathogenic role of androgen excess and potential therapeutic strategies. Molecular Metabolism. 2020.
- Yao K, Bian C, Zhao X. Association of polycystic ovary syndrome with metabolic syndrome and gestational diabetes: Aggravated complication of pregnancy. Experimental and therapeutic medicine. 2017; 14(2): 1271-1276.
- Schauer PR, Burguera B, Ikramuddin S, et al. Effect of laparoscopic roux-en Y gastric bypass on type 2 diabetes mellitus. Ann Surg. 2003; 238(4): 467-84; discussion 84-5.
- Jamal M, Gunay Y, Capper A, et al. Roux-en-Y gastric bypass ameliorates polycystic ovary syndrome and dramatically improves conception rates: A 9-year analysis. Surgery for Obesity and Related Diseases. 2012; 8(4): 440-444.
- Eid GM, Cottam DR, Velcu LM, et al. Effective treatment of polycystic ovarian syndrome with roux-en-Y gastric bypass. Surgery for Obesity and Related Diseases. 2005; 1(2): 77-80.
- Maggard MA, Yermilov I, Li Z, et al. Pregnancy and fertility following bariatric surgery: A systematic review. JAMA. 2008; 300(19): 2286-2296.
- Holst JJ, Madsbad S, Bojsen-Møller KN, et al. Mechanisms in bariatric surgery: Gut hormones, diabetes resolution, and weight loss. Surgery for Obesity and Related Diseases. 2018; 14(5): 708-714.
- Moffett RC, Naughton V. Emerging role of GIP and related gut hormones in fertility and PCOS. Peptides. 2020; 170233.
- Cummings DE, Overduin J, Foster-Schubert KE. Gastric bypass for obesity: Mechanisms of weight loss and diabetes resolution. The Journal of Clinical Endocrinology & Metabolism. 2004; 89(6): 2608-2615.
- Meek CL, Lewis HB, Reimann F, et al. The effect of bariatric surgery on gastrointestinal and pancreatic peptide hormones. Peptides. 2016; 77: 28-37.
- Gissey LC, Mariolo JC, Mingrone G. Intestinal peptide changes after bariatric and minimally invasive surgery: Relation to diabetes remission. Peptides. 2018; 100: 114-122.
- Flatt PR, Day C, Bailey CJ. Bariatric surgery: To treat diabesity. The British Journal of Diabetes & Vascular Disease. 2009; 9(3): 103-107.
- Zhou J, Hao Z, Irwin N, et al. Gastric inhibitory polypeptide (GIP) is selectively decreased in the roux-limb of dietary obese mice after RYGB surgery. PLoS One. 2015; 10(8): e0134728.
- Cummings DE, Weigle DS, Frayo RS, et al. Plasma ghrelin levels after diet-induced weight loss or gastric bypass surgery. N Engl J Med. 2002; 346(21): 1623-1630.
- Mumphrey M, Patterson L, Zheng H, et al. Roux-en-Y gastric bypass surgery increases number but not density of CCK-, GLP-1-, 5-HT-, and neurotensin-expressing enteroendocrine cells in rats. Neurogastroenterology & Motility. 2013; 25(1): e70-e79.
- Christ-Crain M, Stoeckli R, Ernst A, et al. Effect of gastric bypass and gastric banding on proneurotensin levels in morbidly obese patients. The Journal of Clinical Endocrinology & Metabolism. 2006; 91(9): 3544-3547.
- Holdstock C, Zethelius B, Sundbom M, et al. Postprandial changes in gut regulatory peptides in gastric bypass patients. Int J Obes. 2008; 32(11): 1640-1646.
- Skubleny D, Switzer NJ, Gill RS, et al. The impact of bariatric surgery on polycystic ovary syndrome: A systematic review and meta-analysis. Obesity Surg. 2016; 26(1): 169-176.
- Kennedy G, Mitra J. Body weight and food intake as initiating factors for puberty in the rat. J Physiol (Lond). 1963; 166(2): 408-418.
- Heppner KM, Baquero AF, Bennett CM, et al. GLP-1R signaling directly activates arcuate nucleus kisspeptin action in brain slices but does not rescue luteinizing hormone inhibition in ovariectomized mice during negative energy balance. eNeuro. 2017; 4(1): 10.1523/ENEURO.0198-16.2016. eCollection 2017 Jan-Feb.
- Beak SA, Heath MM, Small CJ, et al. Glucagon-like peptide-1 stimulates luteinizing hormone-releasing hormone secretion in a rodent hypothalamic neuronal cell line. J Clin Invest. 1998; 101(6): 1334-1341.
- Outeirino-Iglesias V, Romani-Perez M, Gonzalez-Matias LC, et al. GLP-1 increases preovulatory LH source and the number of mature follicles, as well as synchronizing the onset of puberty in female rats. Endocrinology. 2015; 156(11): 4226-4237.
- Farkas I, Vastagh C, Farkas E, et al. Glucagon-like peptide-1 excites firing and increases GABAergic miniature postsynaptic currents (mPSCs) in gonadotropin-releasing hormone (GnRH) neurons of the male mice via activation of nitric oxide (NO) and suppression of endocannabinoid signaling pathways. Frontiers in cellular neuroscience. 2016; 10: 214.
- Johnson ML, Saffrey MJ, Taylor VJ. Glucagon-like peptide-1 (GLP-1) increases in plasma and colon tissue prior to estrus and circulating levels change with increasing age in reproductively competent wistar rats. Peptides. 2017; 90: 55-62.
- Jensterle M, Janez A, Fliers E, et al. The role of glucagon-like peptide-1 in reproduction: From physiology to therapeutic perspective. Hum Reprod Update. 2019; 25(4): 504-517.
- Gogebakan O, Osterhoff MA, Schuler R, et al. GIP increases adipose tissue expression and blood levels of MCP-1 in humans and links high energy diets to inflammation: A randomised trial. Diabetologia. 2015; 58(8): 1759-1768.
- Gambineri A, Vicennati V, Genghini S, et al. Genetic variation in 11β-hydroxysteroid dehydrogenase type 1 predicts adrenal hyperandrogenism among lean women with polycystic ovary syndrome. The Journal of Clinical Endocrinology & Metabolism. 2006; 91(6): 2295-2302.
- Vrbikova J, Hill M, Bendlova B, et al. Incretin levels in polycystic ovary syndrome. European journal of endocrinology. 2008; 159(2): 121-128.
- Nishiyama Y, Hasegawa T, Fujita S, et al. Incretins modulate progesterone biosynthesis by regulating bone morphogenetic protein activity in rat granulosa cells. J Steroid Biochem Mol Biol. 2018; 178: 82-88.
- Khan D, Vasu S, Moffett RC, et al. Islet distribution of peptide YY and its regulatory role in primary mouse islets and immortalised rodent and human beta-cell function and survival. Mol Cell Endocrinol. 2016; 436: 102-113.
- Comninos AN, Jayasena CN, Dhillo WS. The relationship between gut and adipose hormones, and reproduction. Hum Reprod Update. 2014; 20(2): 153-174.
- Clarke IJ, Backholer K, Tilbrook AJ. Y2 receptor-selective agonist delays the estrogen-induced luteinizing hormone surge in ovariectomized ewes, but y1-receptor-selective agonist stimulates voluntary food intake. Endocrinology. 2005; 146(2): 769-775.
- Baranowska B, Radzikowska M, Wasilewska-Dziubinska E, et al. Neuropeptide Y, leptin, galanin and insulin in women with polycystic ovary syndrome. Gynecological Endocrinology. 1999; 13(5): 344-351.
- Romualdi D, De Marinis L, Campagna G, et al. Alteration of ghrelin–neuropeptide Y network in obese patients with polycystic ovary syndrome: Role of hyperinsulinism. Clin Endocrinol (Oxf). 2008; 69(4): 562-567.
- Korner M, Waser B, Reubi JC. High expression of neuropeptide y receptors in tumors of the human adrenal gland and extra-adrenal paraganglia. Clin Cancer Res. 2004; 10(24): 8426-8433.
- Hill JW, Urban JH, Xu M, et al. Estrogen induces neuropeptide Y (NPY) Y1 receptor gene expression and responsiveness to NPY in gonadotrope-enriched pituitary cell cultures. Endocrinology. 2004; 145(5): 2283-2290.
- Kojima M, Hosoda H, Date Y, et al. Ghrelin is a growth-hormone-releasing acylated peptide from stomach. Nature. 1999; 402(6762): 656-660.
- Pagotto U, Gambineri A, Vicennati V, et al. Plasma ghrelin, obesity, and the polycystic ovary syndrome: Correlation with insulin resistance and androgen levels. The Journal of Clinical Endocrinology & Metabolism. 2002; 87(12): 5625-5629.
- Aghajanova L, Rumman A, Altmae S, et al. Diminished endometrial expression of ghrelin and ghrelin receptor contributes to infertility. Reproductive sciences. 2010; 17(9): 823-832.
- Tena-Sempere M, Aguilar E, Fernandez-Fernandez R, et al. Ghrelin inhibits prolactin secretion in prepubertal rats. Neuroendocrinology. 2004; 79(3): 133-141.
- Carraway R, Leeman SE. Characterization of radioimmunoassayable neurotensin in the rat. its differential distribution in the central nervous system, small intestine, and stomach. J Biol Chem. 1976; 251(22): 7045-7052.
- Thibault D, Albert PR, Pineyro G, et al. Neurotensin triggers dopamine D2 receptor desensitization through a protein kinase C and beta-arrestin1-dependent mechanism. J Biol Chem. 2011; 286(11): 9174-9184.
- Hiradate Y, Inoue H, Kobayashi N, et al. Neurotensin enhances sperm capacitation and acrosome reaction in mice. Biol Reprod. 2014; 91(2): 53, 1-9.
- Craig M, Hales MD, Margaret D, et al. Prevalence of Obesity and Severe Obesity Among Adults: United States, 2017–2018. 2020; https://stacks.cdc.gov/view/cdc/85451. Accessed June 15 2020, 2020.
- Bronstein J, Tawdekar S, Liu Y, et al. Age of onset of polycystic ovarian syndrome in girls may be earlier than previously thought. J Pediatr Adol Gynec. 2011; 24(1): 15-20.
- Inge TH, Garcia V, Daniels S, et al. A multidisciplinary approach to the adolescent bariatric surgical patient. J Pediatr Surg. 2004; 39(3): 442-447.
- Escobar-Morreale HF, Botella-Carretero JI, Alvarez-Blasco F, et al. The polycystic ovary syndrome associated with morbid obesity may resolve after weight loss induced by bariatric surgery. J Clin Endocrinol Metab. 2005; 90(12): 6364-6369.
- Turkmen S, Andreen L, Cengiz Y. Effects of Roux-en-Y gastric bypass surgery on eating behaviour and allopregnanolone levels in obese women with polycystic ovary syndrome. Gynecol Endocrinol. 2015; 31(4): 301-305.
- Jamal M, Gunay Y, Capper A, et al. Roux-en-Y gastric bypass ameliorates polycystic ovary syndrome and dramatically improves conception rates: a 9-year analysis. Surg Obes Relat Dis. 2012; 8(4): 440-444.
- Eid GM, Cottam DR, Velcu LM, et al. Effective treatment of polycystic ovarian syndrome with Roux-en-Y gastric bypass. Surg Obes Relat Dis. 2005; 1(2): 77-80.
